Biology of adult stem cells
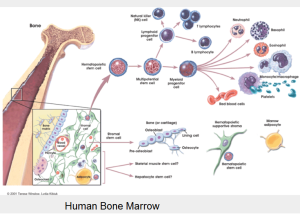
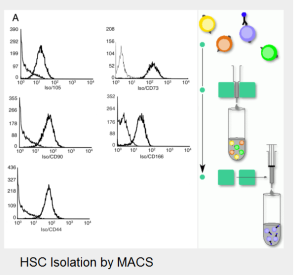
Isolation and characterization of mesenchymal adult bone marrow stem cells (MSC) and hematopoietic stem cells (HSC) from healthy donors and patients with leukemia. Characterization of functional potency and description of stem cell niches. Interaction of stem cells with extracellular matrix and artificial scaffolds.
Mesenchymal stromal cells (MSC) have been shown to be an important component of the hematopoietic stem cell niche. The formation of cobblestone areas (CAF) during long-term culture of HSC on MSC layers is considered a surrogate model for the physiological homing and retention behaviour of HSC within the bone marrow environment. Various cell-cell and cell-matrix interactions are involved to enable the functional characteristics of the niche.
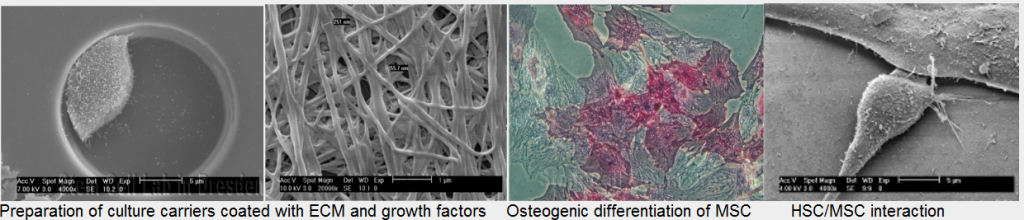
Within the previous project period we could demonstrate that of the presence of extracellular matrix components (ECM) and matrix-bound differentiation factors can influence the potential of MSC to support CAF cells. Culture of HSC within a fibrillar collagen meshwork was found to significantly impact on gene expression profile of HSC. Quantitative expression of genes relevant for cell division, mobilisation and self-renewal was shown to be modulated by an ECM-based culture carrier. Furthermore, the extent of adhesion of HSC to ECM-coated surfaces seems to depend on the choice of the ECM component. Parallel experiments validated a new immobilisation strategy as a valuable tool to create biometic culture substrates combining ECM and bioactive molecules like stem cell factor.
In addition, we could demonstrate that endogenous BMP production can play a role in the spontaneous osteogenic differentiation of MSC in-vitro. Different preparations of extracellular matrix components including a three-dimensional culture system induced significant changes in gene expression profiles of MSC during 14 days of culture. These changes were associated with the frequency of CACF in limiting dilution assays combining MSC on modified substrates and HSC.
Within the upcoming funding period we would like to follow-up on these findings and specifically dissect the in-vitro HSC niche to systematically screen artificial matrices for their suitability as niche substrates.
To address these questions we propose the following work programme:
i) In-situ analysis of signalling molecules that are proposed to be involved in the
hematopoietic niche to support HSC quiescence and/or self-renewal. This will involve functional assays (CAFC) with human primary MSCs as stromal feeder layers. Over-expression of important signalling molecules (Jagged 1, Angiopoietin, SCF, PGE2) in primary MSCs will help to test their potential to support HSCs in culture. Confocal laser scanning fluorescence microscopy and dedicated scanning and transmission electron microscopy techniques will be used to delineate specific candidate molecules involved in the niche-stem cell synapsis.
ii) Creating a reporter cell line from murine MSC to read out which culture conditions favour an elevated expression of HSC supportive factors. Genes that were tested positive for HSC support will be linked to the expression of a reporter molecule (i.e. GFP) to help detect their expression in later culture substrate screens (see point 3). iii) Creating a library of extracellular matrix assemblies (different molecules in different combinations) immobilised via reactive polymer thin films. This set of substrates with different ECM assemblies is then going to be screened using the reporter cell line to highlight surfaces that are ultimately supporting HSC culture by improving stromal cell characteristics.